Oncoral is a novel daily irinotecan chemotherapy in development. Irinotecan chemotherapy has an established potent anti-tumor effect – even in difficult to treat cancers. Oncoral is a daily irinotecan tablet with the potential to offer better patient outcomes with improved safety following the daily dosing at home compared to intravenous high-dose infusions at the hospital. Following successful Phase 1 results, Phase 2 clinical development for Oncoral is in preparation.
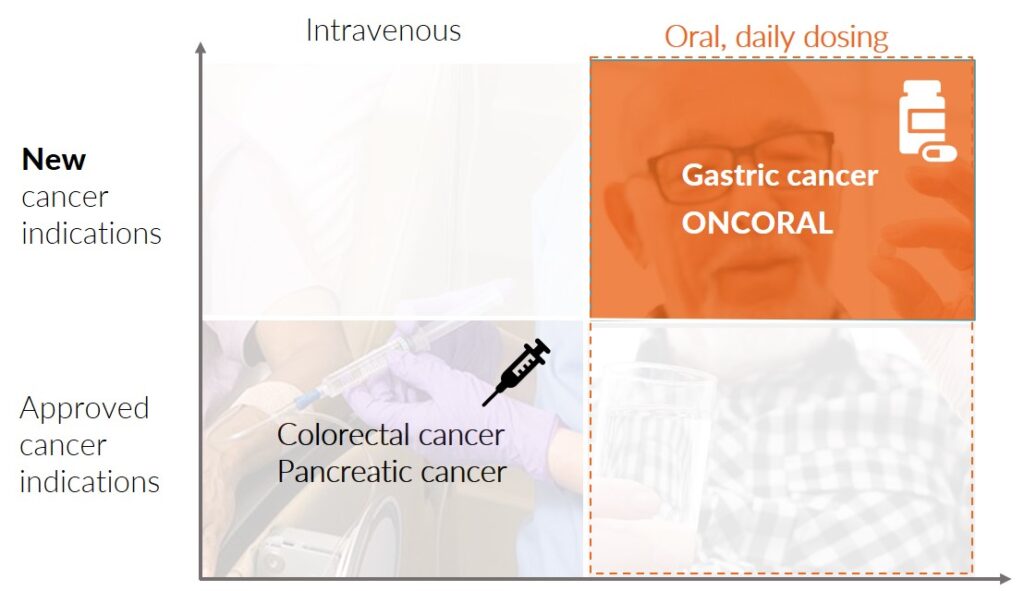
Oncoral is initially being developed for the treatment of gastric cancer in combination with other anti-cancer treatments. Watch presentations about the development plan here:
Irinotecan is a so called antineoplastic agent that after metabolic activation inhibits the enzyme topoisomerase 1, thereby inducing cancer cell death via the prevention of their DNA replication. Irinotecan is converted by carboxylesterases, primarily in the liver, to the active metabolite SN-38 which is 100–1,000 more potent than irinotecan in killing tumor cells.
Data also shows a high conversion rate of irinotecan to the active metabolite SN-38, which has a high anti-tumor activity.
Oncoral – a novel formulation of irinotecan
In addition, the suppressed immune function stemming from the chemotherapy treatment leads to an increased risk of infections and common gastrointestinal adverse events.
Oncoral is a daily irinotecan tablet with the potential to offer better patient outcomes with improved safety. Daily dosing can potentially improve efficacy driven by a more favorable, dosing-related, pharmacokinetic and pharmacodynamic profile. A continuous, low dose regimen also has the potential to reduce the severity of adverse events, infections. and other complications, including dosing flexibility and the possibility of faster discontinuation.
Furthermore, Oncoral has the potential to be combined with other chemotherapies and cancer drugs to potentially enable an all-oral combination option, which may reduce the burden of treatment and make adherence easier.
In addition, home administration can increase convenience for patients and alleviate healthcare staff and equipment allocated to hospital IV administration.
Following successful Phase 1 results, Phase 2 clinical development for Oncoral is in preparation.
A total of 25 patients were enrolled in the first part of the study with Oncoral given as single agent. A further 12 patients were enrolled in the second part of the study where Oncoral was given in the combination study.
Oncoral Phase 1 studies were published in scientific journals.
Kümler et al. Cancer Chemother Pharmacol. 2019 Aug;84(2):441-446 (link)
Kümler et al. Cancer Chemother Pharmacol. 2019 Jan;83(1):169-178. (link)
| Phase 1 results – single agent study (publ. 2019) | Phase 1 results – combination study (publ. 2019) |
|---|---|
| Study: Dose escalating, open label, single center 25 patients with metastatic or unresectable solid tumors | Study: Open label, single center 14 patients with metastatic or unresectable solid tumors |
| Hematological toxicities were few and all mild (grade 1) to moderate (grade 2) | The combination of Oncoral with another oral chemotherapy, demonstrated reassuring tolerability |
| Pharmaco-Kinetic (PK) data showed consistent daily exposures during treatment at days 1 and 14 with no drug accumulation | |
| The active metabolite, SN-38, interpatient variability was in the same range as for IV administration |
The plan is to design and conduct a Phase 2 study on Oncoral in combination with other anti-cancer drugs (potentially oral) in patients who are irinotecan-naive and HER2 negative with unresectable or metastatic gastric cancer.
You can read more about the Phase 2 plans here
Please note that Oncoral is an investigational medicinal product and is not yet approved for use by regulatory authorities in any jurisdiction.